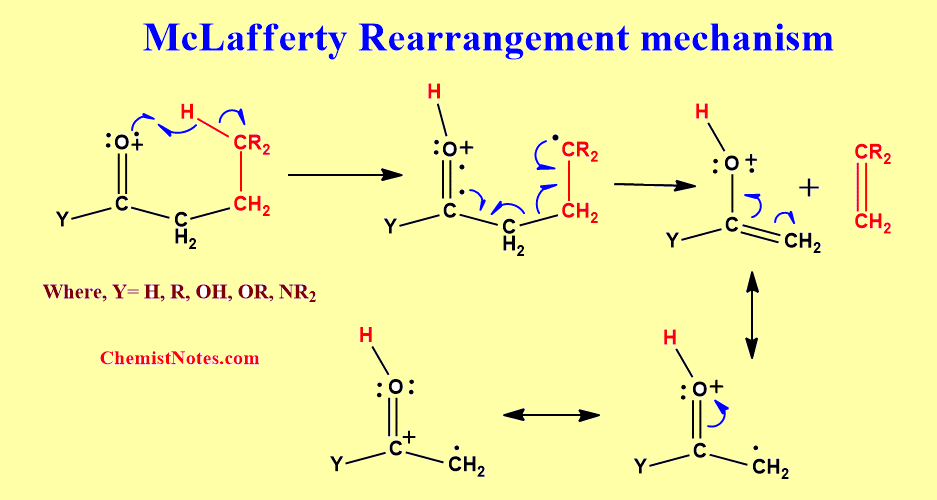
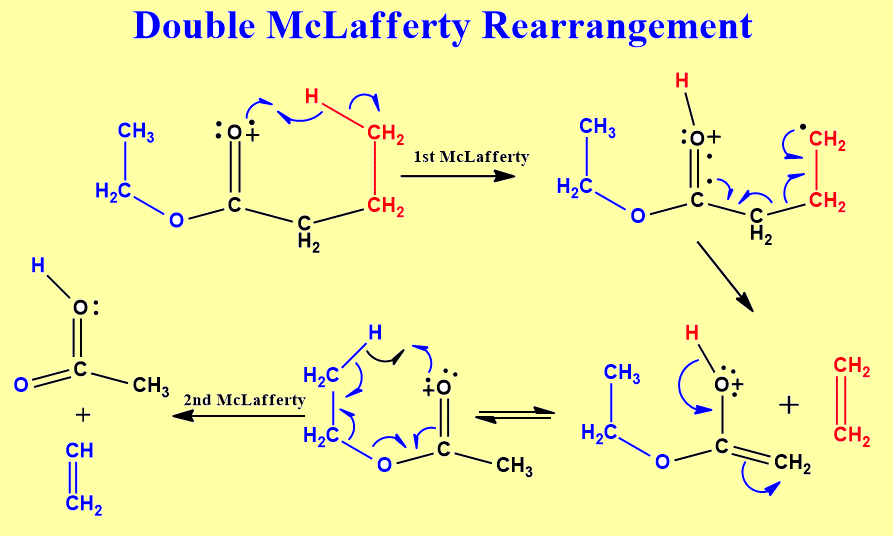
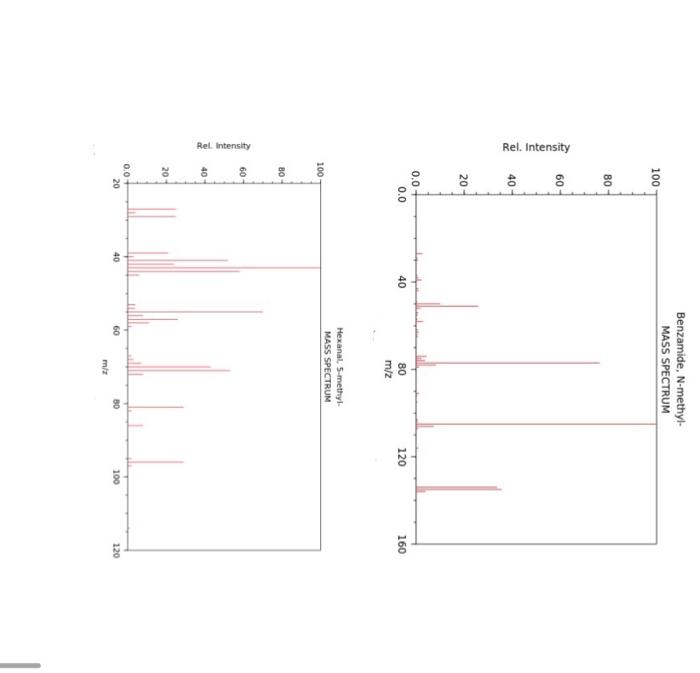
The McLafferty rearrangement is a reaction observed in mass spectrometry during the fragmentation or dissociation of organic molecules. It is sometimes found that a molecule containing a keto-group undergoes β-cleavage, with the gain of the γ-hydrogen atom, as first reported by Anthony Nicholson working in the Division of Chemical Physics at the CSIRO in Australia. This rearrangement may take place by a radical or ionic mechanism.
The reaction
A description of the reaction was later published by the American chemist Fred McLafferty in 1959 leading to his name being associated with the process.
See also
- The Type II Norrish reaction is the equivalent photochemical process
- α-cleavage
References
Further reading
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "McLafferty rearrangement". doi:10.1351/goldbook.M03772
External links
- Fred McLafferty Faculty Webpage at Cornell University